Speed when moving with constant acceleration. Physics lesson on "Acceleration. Kinematics - it's easy"
In this lesson, the topic of which is: “Equation of motion with constant acceleration. Forward movement,” we will remember what movement is, what it happens. Let’s also remember what acceleration is, consider the equation of motion with constant acceleration and how to use it to determine the coordinates of a moving body. Let's consider an example of a task for consolidating material.
The main task of kinematics is to determine the position of the body at any time. The body can be at rest, then its position will not change (see Fig. 1).
Rice. 1. Body at rest
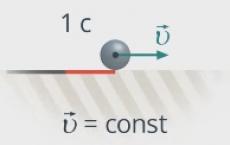
A body can move in a straight line at a constant speed. Then its movement will change uniformly, that is, equally over equal periods of time (see Fig. 2).

Rice. 2. Movement of a body when moving at a constant speed
Movement, speed multiplied by time, we have been able to do this for a long time. A body can move with constant acceleration; consider such a case (see Fig. 3).

Rice. 3. Body motion with constant acceleration
Acceleration
|
Acceleration is the change in speed per unit time(see Fig. 4) :
Rice. 4. Acceleration Speed is a vector quantity, therefore the change in speed, i.e. the difference between the vectors of the final and initial speed, is a vector. Acceleration is also a vector, directed in the same direction as the vector of the speed difference (see Fig. 5).
We are considering linear motion, so we can select a coordinate axis along the straight line along which the motion occurs, and consider the projections of the velocity and acceleration vectors onto this axis:
|
Then its speed changes uniformly: (if its initial speed was zero). How to find the displacement now? It is impossible to multiply speed by time: the speed was constantly changing; which one to take? How to determine where the body will be at any moment during such a movement - today we will solve this problem.
Let’s immediately define the model: we are considering the rectilinear translational motion of a body. In this case, we can use the material point model. Acceleration is directed along the same straight line along which the material point moves (see Fig. 6).

Forward movement
|
Translational motion is a movement in which all points of the body move the same way: at the same speed, making the same movement (see Fig. 7).
Rice. 7. Forward movement How else could it be? Wave your hand and observe: it is clear that the palm and shoulder moved differently. Look at the Ferris wheel: the points near the axis hardly move, but the cabins move at different speeds and along different trajectories (see Fig. 8).
Rice. 8. Movement of selected points on the Ferris wheel Look at a moving car: if you do not take into account the rotation of the wheels and the movement of engine parts, all points of the car move equally, we consider the movement of the car to be translational (see Fig. 9).
Rice. 9. Car movement Then there is no point in describing the movement of each point; you can describe the movement of one. We consider a car to be a material point. Please note that during translational movement, the line connecting any two points of the body during movement remains parallel to itself (see Fig. 10).
Rice. 10. Position of the line connecting two points |
The car drove straight for an hour. At the beginning of the hour his speed was 10 km/h, and at the end - 100 km/h (see Fig. 11).

Rice. 11. Drawing for the problem
The speed changed uniformly. How many kilometers did the car travel?
Let us analyze the condition of the problem.
The speed of the car changed uniformly, that is, its acceleration was constant throughout the journey. Acceleration by definition is equal to:
![]()
The car was driving straight, so we can consider its movement in projection onto one coordinate axis:
![]()
Let's find the displacement.
Increasing speed example
|
Nuts are placed on the table, one nut per minute. It’s clear: no matter how many minutes pass, so many nuts will appear on the table. Now let’s imagine that the rate of placing nuts increases uniformly from zero: the first minute no nuts are placed, the second minute they put one nut, then two, three, and so on. How many nuts will be on the table after some time? It is clear that it is less than if the maximum speed was always maintained. Moreover, it is clearly visible that it is 2 times less (see Fig. 12).
Rice. 12. Number of nuts at different laying speeds It’s the same with uniformly accelerated motion: let’s say that at first the speed was zero, but at the end it became equal (see Fig. 13).
Rice. 13. Change speed If the body were constantly moving at such a speed, its displacement would be equal to , but since the speed increased uniformly, it would be 2 times less. |
We know how to find displacement during UNIFORM movement: . How to work around this problem? If the speed does not change much, then the movement can be approximately considered uniform. The change in speed will be small over a short period of time (see Fig. 14).

Rice. 14. Change speed
Therefore, we divide the travel time T into N small segments of duration (see Fig. 15).

Rice. 15. Splitting a period of time
Let's calculate the displacement at each time interval. The speed increases at each interval by:
On each segment we will consider the movement to be uniform and the speed approximately equal to the initial speed for a given period of time. Let's see if our approximation will lead to an error if we assume the motion to be uniform over a short interval. The maximum error will be:
and the total error for the entire journey -> . For large N we assume the error is close to zero. We will see this on the graph (see Fig. 16): there will be an error at each interval, but the total error with a sufficiently large number of intervals will be negligible.

Rice. 16. Interval error
So, each subsequent speed value is the same amount greater than the previous one. From algebra we know that this is an arithmetic progression with a progression difference:
The path in the sections (with uniform rectilinear motion (see Fig. 17) is equal to:

Rice. 17. Consideration of areas of body movement
On the second section:
On the nth section the path is:
Arithmetic progression
|
Arithmetic progression is a number sequence in which each subsequent number differs from the previous one by the same amount. An arithmetic progression is specified by two parameters: the initial term of the progression and the difference of the progression. Then the sequence is written like this: The sum of the first terms of an arithmetic progression is calculated using the formula:
|
Let's sum up all the paths. This will be the sum of the first N terms of the arithmetic progression:
Since we have divided the movement into many intervals, we can assume that then:
We had many formulas, and in order not to get confused, we did not write the x indices each time, but considered everything in projection onto the coordinate axis.
So, we have obtained the main formula for uniformly accelerated motion: displacement during uniformly accelerated motion in time T, which, along with the definition of acceleration (change in speed per unit time), we will use to solve problems:
![]()
![]()
We were working on solving a problem about a car. Let's substitute numbers into the solution and get the answer: the car traveled 55.4 km.
Mathematical part of solving the problem
We figured out the movement. How to determine the coordinate of a body at any moment in time?
By definition, the movement of a body over time is a vector, the beginning of which is at the initial point of movement, and the end is at the final point at which the body will be after time. We need to find the coordinate of the body, so we write an expression for the projection of displacement onto the coordinate axis (see Fig. 18):

Rice. 18. Motion projection
Let's express the coordinate:
That is, the coordinate of the body at the moment of time is equal to the initial coordinate plus the projection of the movement that the body made during the time. We have already found the projection of displacement during uniformly accelerated motion, all that remains is to substitute and write:
![]()
This is the equation of motion with constant acceleration. It allows you to find out the coordinates of a moving material point at any time. It is clear that we choose the moment of time within the interval when the model works: the acceleration is constant, the movement is rectilinear.
Why the equation of motion cannot be used to find a path
|
In what cases can we consider movement modulo equal to path? When a body moves along a straight line and does not change direction. For example, with uniform rectilinear motion, we do not always clearly define whether we are finding a path or a displacement; they still coincide. With uniformly accelerated motion, the speed changes. If the speed and acceleration are directed in opposite directions (see Fig. 19), then the velocity modulus decreases, and at some point it will become equal to zero and the speed will change direction, that is, the body will begin to move in the opposite direction.
Rice. 19. Velocity modulus decreases And then, if at a given moment in time the body is at a distance of 3 m from the beginning of observation, then its displacement is equal to 3 m, but if the body first traveled 5 m, then turned around and traveled another 2 m, then the path will be equal to 7 m. And how How can you find it if you don’t know these numbers? You just need to find the moment when the speed is zero, that is, when the body turns around, and find the path to and from this point (see Fig. 20).
Rice. 20. The moment when the speed is 0 |
Bibliography
- Sokolovich Yu.A., Bogdanova G.S. Physics: A reference book with examples of problem solving. - 2nd edition repartition. - X.: Vesta: Ranok Publishing House, 2005. - 464 p.
- Landsberg G.S. Elementary physics textbook; v.1. Mechanics. Heat. Molecular physics - M.: Publishing house "Science", 1985.
- Internet portal “kaf-fiz-1586.narod.ru” ()
- Internet portal “Study - Easy” ()
- Internet portal "Knowledge Hypermarket" ()
Homework
- What is an arithmetic progression?
- What kind of movement is called translational?
- What is a vector quantity characterized by?
- Write down the formula for acceleration through a change in speed.
- What is the form of the equation of motion with constant acceleration?
- The acceleration vector is directed towards the movement of the body. How will the body change its speed?
Rectilinear motion with constant acceleration is called uniformly accelerated if the velocity module increases with time, or uniformly decelerated if it decreases.
An example of accelerated motion would be a flower pot falling from the balcony of a low building. At the beginning of the fall, the speed of the pot is zero, but in a few seconds it manages to increase to tens of m/s. An example of slow motion is the movement of a stone thrown vertically upward, the speed of which is initially high, but then gradually decreases to zero at the top point of the trajectory. If we neglect the force of air resistance, then the acceleration in both of these cases will be the same and equal to the acceleration of free fall, which is always directed vertically downward, denoted by the letter g and equal to approximately 9.8 m/s2.
The acceleration due to gravity, g, is caused by the gravitational force of the Earth. This force accelerates all bodies moving towards the earth and slows down those moving away from it.
where v is the speed of the body at time t, from where, after simple transformations, we obtain equation for speed when moving with constant acceleration: v = v0 + at
8. Equations of motion with constant acceleration.
To find the equation for speed during linear motion with constant acceleration, we will assume that at time t=0 the body had an initial speed v0. Since the acceleration a is constant, the following equation is valid for any time t:
where v is the speed of the body at time t, from where, after simple transformations, we obtain the equation for speed when moving with constant acceleration: v = v0 + at
To derive an equation for the path traveled during rectilinear motion with constant acceleration, we first construct a graph of speed versus time (5.1). For a>0, the graph of this dependence is shown on the left in Fig. 5 (blue straight line). As we established in §3, the movement accomplished during time t can be determined by calculating the area under the velocity versus time curve between moments t=0 and t. In our case, the figure under the curve, bounded by two vertical lines t = 0 and t, is a trapezoid OABC, the area of which S, as is known, is equal to the product of half the sum of the lengths of the bases OA and CB and the height OC:
As can be seen in Fig. 5, OA = v0, CB = v0 + at, and OC = t. Substituting these values into (5.2), we obtain the following equation for the displacement S made in time t during rectilinear motion with constant acceleration a at an initial speed v0:
It is easy to show that formula (5.3) is valid not only for motion with acceleration a>0, for which it was derived, but also in those cases when a<0. На рис.5 справа красными линиями показаны графики зависимости S при положительных (верх) и отрицательных (низ) значениях a, построенные по формуле (5.3) для различных величин v0. Видно, что в отличие от равномерного движения (см. рис. 3), график зависимости перемещения от времени является параболой, а не прямой, показанной для сравнения пунктирной линией.
9. Free fall of bodies. Motion with constant acceleration due to gravity.
Free fall of bodies is the fall of bodies to the Earth in the absence of air resistance (in vacuum)
The acceleration with which bodies fall to the Earth is called the acceleration of gravity. The free fall acceleration vector is indicated by the symbol; it is directed vertically downward. At different points on the globe, depending on the geographic latitude and altitude above sea level, the numerical value of g is not the same, varying from approximately 9.83 m/s2 at the poles to 9.78 m/s2 at the equator. At the latitude of Moscow g = 9.81523 m/s2. Usually, if high accuracy is not required in the calculations, then the numerical value of g at the Earth's surface is taken equal to 9.8 m/s2 or even 10 m/s2.
A simple example of free fall is a body falling from a certain height h without an initial speed. Free fall is a linear motion with constant acceleration.
An ideal free fall is possible only in a vacuum, where there is no air resistance, and regardless of mass, density and shape, all bodies fall equally quickly, i.e. at any moment in time the bodies have the same instantaneous speeds and accelerations.
All formulas for uniformly accelerated motion are applicable to freely falling bodies.
The magnitude of the speed during free fall of a body at any time:
body movement:
In this case, instead of acceleration a, the acceleration of gravity g = 9.8 m/s2 is introduced into the formulas for uniformly accelerated motion.
10. Movement of bodies. FORWARD MOTION OF A RIGID BODY
The translational motion of a rigid body is such a motion in which every straight line, invariably connected with the body, moves parallel to itself. To do this, it is enough that two non-parallel lines connected to the body move parallel to themselves. During translational motion, all points of the body describe identical, parallel trajectories and have the same speeds and accelerations at any given time. Thus, the translational motion of a body is determined by the movement of one of its points O.
In the general case, translational motion occurs in three-dimensional space, but its main feature - maintaining parallelism of any segment to itself - remains in force.
For example, an elevator car moves forward. Also, to a first approximation, the Ferris wheel cabin makes translational motion. However, strictly speaking, the movement of the Ferris wheel cabin cannot be considered progressive. If a body moves translationally, then to describe its movement it is enough to describe the movement of an arbitrary point (for example, the movement of the center of mass of the body).
If the bodies that make up a closed mechanical system interact with each other only through the forces of gravity and elasticity, then the work of these forces is equal to the change in the potential energy of the bodies, taken with the opposite sign: A = –(E р2 – E р1).
According to the kinetic energy theorem, this work is equal to the change in the kinetic energy of bodies
Hence
Or E k 1 + E p 1 = E k 2 + E p 2.
The sum of kinetic and potential energy of bodies that make up a closed system and interact with each other through gravitational and elastic forces remains unchanged.
This statement expresses the law of conservation of energy in mechanical processes. It is a consequence of Newton's laws. The sum E = E k + E p is called total mechanical energy. The law of conservation of mechanical energy is satisfied only when bodies in a closed system interact with each other by conservative forces, that is, forces for which the concept of potential energy can be introduced.
The mechanical energy of a closed system of bodies does not change if only conservative forces act between these bodies. Conservative forces are those forces whose work along any closed trajectory is equal to zero. Gravity is one of the conservative forces.
In real conditions, moving bodies are almost always acted upon, along with gravitational forces, elastic forces and other conservative forces, by frictional forces or environmental resistance forces.
The friction force is not conservative. The work done by the friction force depends on the length of the path.
If friction forces act between the bodies that make up a closed system, then mechanical energy is not conserved. Part of the mechanical energy is converted into internal energy of bodies (heating).
During any physical interactions, energy neither appears nor disappears. It just changes from one form to another.
One of the consequences of the law of conservation and transformation of energy is the statement about the impossibility of creating a “perpetual motion machine” (perpetuum mobile) - a machine that could do work indefinitely without consuming energy.
History stores a considerable number of “perpetual motion” projects. In some of them, the mistakes of the “inventor” are obvious, in others these mistakes are masked by the complex design of the device, and it can be very difficult to understand why this machine will not work. Fruitless attempts to create a “perpetual motion machine” continue in our time. All these attempts are doomed to failure, since the law of conservation and transformation of energy “prohibits” obtaining work without expending energy.
31. Basic principles of molecular kinetic theory and their justification.
All bodies consist of molecules, atoms and elementary particles that are separated by spaces, move randomly and interact with each other.
Kinematics and dynamics help us describe the movement of a body and determine the force that causes this movement. However, a mechanic cannot answer many questions. For example, what are bodies made of? Why do many substances become liquid when heated and then evaporate? And, in general, what is temperature and heat?
The ancient Greek philosopher Democritus tried to answer similar questions 25 centuries ago. Without performing any experiments, he came to the conclusion that bodies only seem solid to us, but in fact they consist of tiny particles separated by emptiness. Considering that it was impossible to crush these particles, Democritus called them atoms, which translated from Greek means indivisible. He also suggested that atoms can be different and are in constant motion, but we do not see this, because they are very small.
M.V. made a great contribution to the development of molecular kinetic theory. Lomonosov. Lomonosov was the first to suggest that heat reflects the movement of atoms in a body. In addition, he introduced the concept of simple and complex substances, the molecules of which consist of identical and different atoms, respectively.
Molecular physics or molecular kinetic theory is based on certain ideas about the structure of matter
Thus, according to the atomic theory of the structure of matter, the smallest particle of a substance that retains all its chemical properties is a molecule. Even large molecules, consisting of thousands of atoms, are so small that they cannot be seen with a light microscope. Numerous experiments and theoretical calculations show that the size of atoms is about 10 -10 m. The size of a molecule depends on how many atoms it consists of and how they are located relative to each other.
Molecular kinetic theory is the study of the structure and properties of matter based on the idea of the existence of atoms and molecules as the smallest particles of chemical substances.
The molecular kinetic theory is based on three main principles:
1. All substances - liquid, solid and gaseous - are formed from the smallest particles - molecules, which themselves consist of atoms ("elementary molecules"). The molecules of a chemical substance can be simple or complex, i.e. consist of one or more atoms. Molecules and atoms are electrically neutral particles. Under certain conditions, molecules and atoms can acquire additional electrical charge and become positive or negative ions.
2. Atoms and molecules are in continuous chaotic motion.
3. Particles interact with each other by forces that are electrical in nature. The gravitational interaction between particles is negligible.
The most striking experimental confirmation of the ideas of the molecular kinetic theory about the random movement of atoms and molecules is Brownian motion. This is the thermal movement of tiny microscopic particles suspended in a liquid or gas. It was discovered by the English botanist R. Brown in 1827. Brownian particles move under the influence of random impacts of molecules. Due to the chaotic thermal motion of molecules, these impacts never balance each other. As a result, the speed of a Brownian particle changes randomly in magnitude and direction, and its trajectory is a complex zigzag curve.
The constant chaotic movement of the molecules of a substance is also manifested in another easily observable phenomenon - diffusion. Diffusion is the phenomenon of penetration of two or more contacting substances into each other. The process occurs most quickly in gas.
The random chaotic movement of molecules is called thermal motion. The kinetic energy of thermal motion increases with increasing temperature.
A mole is an amount of substance containing the same number of particles (molecules) as there are atoms in 0.012 kg of carbon 12 C. A carbon molecule consists of one atom.
32. Mass of molecules, relative molecular mass of molecules. 33. Molar mass of molecules. 34. Amount of substance. 35. Avogadro's constant.
In molecular kinetic theory, the amount of matter is considered to be proportional to the number of particles. The unit of quantity of a substance is called a mole (mole).
A mole is an amount of substance containing the same number of particles (molecules) as there are atoms in 0.012 kg (12 g) of carbon 12 C. A carbon molecule consists of one atom.
One mole of a substance contains a number of molecules or atoms equal to Avogadro's constant.
Thus, one mole of any substance contains the same number of particles (molecules). This number is called Avogadro's constant N A: N A = 6.02·10 23 mol –1.
Avogadro's constant is one of the most important constants in molecular kinetic theory.
The amount of substance ν is defined as the ratio of the number N of particles (molecules) of the substance to Avogadro’s constant N A:
Molar mass, M, is the ratio of the mass m of a given sample of a substance to the amount n of the substance contained in it:
which is numerically equal to the mass of a substance taken in the amount of one mole. Molar mass in the SI system is expressed in kg/mol.
Thus, the relative molecular or atomic mass of a substance is the ratio of the mass of its molecule and atom to 1/12 the mass of a carbon atom.
36. Brownian motion.
Many natural phenomena indicate the chaotic movement of microparticles, molecules and atoms of matter. The higher the temperature of the substance, the more intense this movement. Therefore, the heat of a body is a reflection of the random movement of its constituent molecules and atoms.
Proof that all atoms and molecules of a substance are in constant and random motion can be diffusion - the interpenetration of particles of one substance into another.
Thus, the smell quickly spreads throughout the room even in the absence of air movement. A drop of ink quickly turns the entire glass of water uniformly black.
Diffusion can also be detected in solids if they are pressed tightly together and left for a long time. The phenomenon of diffusion demonstrates that microparticles of a substance are capable of spontaneous movement in all directions. This movement of microparticles of a substance, as well as its molecules and atoms, is called thermal movement.
BROWNIAN MOTION - random movement of tiny particles suspended in a liquid or gas, occurring under the influence of impacts from environmental molecules; discovered by R. Brown in 1827
Observations show that Brownian motion never stops. In a drop of water (if you do not allow it to dry), the movement of grains can be observed for many days, months, years. It does not stop either in summer or winter, neither day nor night.
The reason for Brownian motion lies in the continuous, never-ending movement of the molecules of the liquid in which the grains of the solid are located. Of course, these grains are many times larger than the molecules themselves, and when we see the movement of the grains under a microscope, we should not think that we are seeing the movement of the molecules themselves. Molecules cannot be seen with an ordinary microscope, but we can judge their existence and movement by the impacts they produce, pushing grains of a solid body and causing them to move.
The discovery of Brownian motion was of great importance for the study of the structure of matter. It showed that bodies really consist of individual particles - molecules and that the molecules are in continuous random motion.
An explanation of Brownian motion was given only in the last quarter of the 19th century, when it became obvious to many scientists that the motion of a Brownian particle is caused by random impacts of molecules of the medium (liquid or gas) undergoing thermal motion. On average, the molecules of the medium impact a Brownian particle from all directions with equal force, however, these impacts never exactly cancel each other out, and as a result, the speed of the Brownian particle varies randomly in magnitude and direction. Therefore, the Brownian particle moves along a zigzag path. Moreover, the smaller the size and mass of a Brownian particle, the more noticeable its movement becomes.
Thus, the analysis of Brownian motion laid the foundations of the modern molecular kinetic theory of the structure of matter.
37. Forces of interaction between molecules. 38. Structure of gaseous substances. 39. Structure of liquid substances. 40. Structure of solids.
The distance between molecules and the forces acting between them determine the properties of gaseous, liquid and solid bodies.
We are accustomed to the fact that liquid can be poured from one vessel to another, and gas quickly fills the entire volume provided to it. Water can only flow along the riverbed, and the air above it knows no boundaries.
There are intermolecular attractive forces between all molecules, the magnitude of which decreases very quickly as the molecules move away from each other, and therefore at a distance equal to several molecular diameters they do not interact at all.
Thus, between liquid molecules located almost close to each other, attractive forces act, preventing these molecules from scattering in different directions. On the contrary, the insignificant forces of attraction between gas molecules are not able to hold them together, and therefore gases can expand, filling the entire volume provided to them. The existence of intermolecular attractive forces can be verified by performing a simple experiment - pressing two lead bars against each other. If the contact surfaces are sufficiently smooth, the bars will stick together and will be difficult to separate.
However, intermolecular attractive forces alone cannot explain all the differences between the properties of gaseous, liquid and solid substances. Why, for example, is it very difficult to reduce the volume of a liquid or solid, but it is relatively easy to compress a balloon? This is explained by the fact that between molecules there are not only attractive forces, but also intermolecular repulsive forces, which act when the electron shells of the atoms of neighboring molecules begin to overlap. It is these repulsive forces that prevent one molecule from penetrating into a volume already occupied by another molecule.
When no external forces act on a liquid or solid body, the distance between their molecules is such that the resultant forces of attraction and repulsion are zero. If you try to reduce the volume of a body, the distance between the molecules decreases, and the resulting increased repulsive forces begin to act from the side of the compressed body. On the contrary, when a body is stretched, the elastic forces that arise are associated with a relative increase in the forces of attraction, because When molecules move away from each other, the repulsive forces fall much faster than the attractive forces.
Gas molecules are located at distances tens of times greater than their sizes, as a result of which these molecules do not interact with each other, and therefore gases are much more easily compressed than liquids and solids. Gases do not have any specific structure and are a collection of moving and colliding molecules.
A liquid is a collection of molecules that are almost closely adjacent to each other. Thermal motion allows a liquid molecule to change its neighbors from time to time, jumping from one place to another. This explains the fluidity of liquids.
Atoms and molecules of solids are deprived of the ability to change their neighbors, and their thermal motion is only small fluctuations relative to the position of neighboring atoms or molecules. The interaction between atoms can lead to the fact that a solid becomes a crystal, and the atoms in it occupy positions at the sites of the crystal lattice. Since the molecules of solid bodies do not move relative to their neighbors, these bodies retain their shape.
41. Ideal gas in molecular kinetic theory.
An ideal gas is a model of a rarefied gas in which interactions between molecules are neglected. The forces of interaction between molecules are quite complex. At very short distances, when molecules come close to each other, large repulsive forces act between them. At large or intermediate distances between molecules, relatively weak attractive forces act. If the distances between molecules are on average large, which is observed in a fairly rarefied gas, then the interaction manifests itself in the form of relatively rare collisions of molecules with each other when they fly close. In an ideal gas, the interaction of molecules is completely neglected.
42. Gas pressure in molecular kinetic theory.
An ideal gas is a model of a rarefied gas in which interactions between molecules are neglected.
The pressure of an ideal gas is proportional to the product of the concentration of molecules and their average kinetic energy.
Gas surrounds us on all sides. Anywhere on earth, even under water, we carry a part of the atmosphere, the lower layers of which are compressed under the influence of gravity from the upper ones. Therefore, by measuring atmospheric pressure we can judge what is happening high above us and predict the weather.
43. The average value of the squared speed of molecules of an ideal gas.
44. Derivation of the basic equation of the molecular kinetic theory of gas. 45. Derivation of a formula relating pressure and average kinetic energy of gas molecules.
Pressure p on a given surface area is the ratio of the force F acting perpendicular to this surface to the area S of its given area
The SI unit of pressure is Pascal (Pa). 1 Pa = 1 N/m2.
Let us find the force F with which a molecule of mass m0 acts on the surface from which it rebounds. When reflected from a surface, lasting a period of time Dt, the component of the velocity of the molecule perpendicular to this surface, vy, changes to the inverse (-vy). Therefore, when reflected from the surface, the molecule acquires momentum, 2m0vy, and therefore, according to Newton’s third law, 2m0vy = FDt, from which:
Formula (22.2) makes it possible to calculate the force with which one gas molecule presses on the wall of the vessel during the interval Dt. To determine the average force of gas pressure, for example, in one second, it is necessary to find how many molecules will be reflected per second from a surface area of area S, and it is also necessary to know the average speed vy of molecules moving in the direction of a given surface.
Let there be n molecules per unit volume of gas. Let's simplify our task by assuming that all gas molecules move at the same speed, v. In this case, 1/3 of all molecules move along the Ox axis, and the same amount along the Oy and Oz axis (see Fig. 22c). Let half of the molecules moving along the Oy axis move towards wall C, and the rest - in the opposite direction. Then, obviously, the number of molecules per unit volume rushing towards wall C will be n/6.
Let us now find the number of molecules that hit a surface area of area S (shaded in Fig. 22c) in one second. Obviously, in 1 s those molecules that move towards it and are at a distance not greater than v will have time to reach the wall. Therefore, 1/6 of all molecules located in the rectangular parallelepiped highlighted in Fig. will hit this area of the surface. 22c, the length of which is v, and the area of the end faces is S. Since the volume of this parallelepiped is Sv, the total number N of molecules hitting a section of the wall surface in 1 s will be equal to:
Using (22.2) and (22.3), we can calculate the impulse that, in 1 s, imparted to the gas molecules a section of the wall surface of area S. This impulse will be numerically equal to the gas pressure force, F:
whence, using (22.1), we obtain the following expression relating the gas pressure and the average kinetic energy of the translational motion of its molecules:
where E CP is the average kinetic energy of ideal gas molecules. Formula (22.4) is called the basic equation of the molecular kinetic theory of gases.
46. Thermal equilibrium. 47. Temperature. Temperature change. 48. Instruments for measuring temperature.
Thermal equilibrium between bodies is possible only when their temperature is the same.
By touching any object with our hand, we can easily determine whether it is warm or cold. If the temperature of an object is lower than the temperature of the hand, the object appears cold, and if, on the contrary, it appears warm. If you hold a cold coin in your fist, the warmth of the hand will begin to heat the coin, and after some time its temperature will become equal to the temperature of the hand, or, as they say, thermal equilibrium will occur. Therefore, temperature characterizes the state of thermal equilibrium of a system of two or more bodies having the same temperature.
Temperature, along with gas volume and pressure, are macroscopic parameters. Thermometers are used to measure temperature. Some of them record changes in the volume of liquid when heated, others record changes in electrical resistance, etc. The most common is the Celsius temperature scale, named after the Swedish physicist A. Celsius. To obtain the Celsius temperature scale for a liquid thermometer, it is first immersed in melting ice and the position of the end of the column is noted, and then in boiling water. The segment between these two positions of the column is divided into 100 equal parts, assuming that the temperature of melting ice corresponds to zero degrees Celsius (o C), and the temperature of boiling water is 100 o C.
49. Average kinetic energy of gas molecules at thermal equilibrium.
The basic equation of molecular kinetic theory (22.4) relates gas pressure, concentration of molecules and their average kinetic energy. However, the average kinetic energy of molecules is, as a rule, unknown, although the results of many experiments indicate that the speed of molecules increases with increasing temperature (see, for example, Brownian motion in §20). The dependence of the average kinetic energy of gas molecules on its temperature can be obtained from the law discovered by the French physicist J. Charles in 1787.
50. Gases in a state of thermal equilibrium (describe the experiment).
51. Absolute temperature. 52. Absolute temperature scale. 53. Temperature is a measure of the average kinetic energy of molecules.
The dependence of the average kinetic energy of gas molecules on its temperature can be obtained from the law discovered by the French physicist J. Charles in 1787.
According to Charles’s law, if the volume of a given mass of gas does not change, its pressure pt depends linearly on temperature t:
where t is the gas temperature measured in o C, and p 0 is the gas pressure at a temperature of 0 o C (see Fig. 23b). Thus, from Charles’ law it follows that the pressure of a gas occupying a constant volume is proportional to the sum (t + 273 o C). On the other hand, it follows from (22.4) that if the concentration of molecules is constant, i.e. the volume occupied by the gas does not change, then the gas pressure must be proportional to the average kinetic energy of the molecules. This means that the average kinetic energy, E SR of gas molecules, is simply proportional to the value (t + 273 o C):
where b is a constant coefficient, the value of which we will determine later. From (23.2) it follows that the average kinetic energy of molecules will become equal to zero at -273 o C. Based on this, the English scientist W. Kelvin in 1848 proposed using an absolute temperature scale, the zero temperature in which would correspond to -273 o C, and every degree of temperature would be equal to a degree on the Celsius scale. Thus, absolute temperature, T, is related to temperature, t, measured in Celsius, as follows:
The SI unit of absolute temperature is Kelvin (K).
Taking into account (23.3), equation (23.2) is transformed into:
substituting which into (22.4), we obtain the following:
To get rid of the fraction in (23.5), we replace 2b/3 with k, and instead of (23.4) and (23.5) we get two very important equations:
where k is Boltzmann’s constant, named after L. Boltzmann. Experiments have shown that k=1.38.10 -23 J/K. Thus, the pressure of a gas and the average kinetic energy of its molecules are proportional to its absolute temperature.
54. Dependence of gas pressure on the concentration of its molecules and temperature.
In most cases, when a gas transitions from one state to another, all its parameters change - temperature, volume and pressure. This happens when gas is compressed under a piston in an internal combustion engine cylinder, causing the gas temperature and pressure to increase and its volume to decrease. However, in some cases, changes in one of the gas parameters are relatively small or even absent. Such processes, where one of the three parameters - temperature, pressure or volume remains unchanged, are called isoprocesses, and the laws that describe them are called gas laws.
55. Measuring the speed of gas molecules. 56. Stern experiment.
First of all, let us clarify what is meant by the speed of molecules. Let us recall that due to frequent collisions, the speed of each individual molecule changes all the time: the molecule moves sometimes quickly, sometimes slowly, and for some time (for example, one second) the speed of the molecule takes on many different values. On the other hand, at any moment in the enormous number of molecules that make up the volume of gas under consideration, there are molecules with very different velocities. Obviously, to characterize the state of the gas, we must talk about some average speed. We can assume that this is the average value of the speed of one of the molecules over a sufficiently long period of time or that this is the average value of the speeds of all gas molecules in a given volume at some point in time.
There are various ways to determine the speed of movement of molecules. One of the simplest is the method implemented in 1920 in the Stern experiment.
Rice. 390. When the space under glass A is filled with hydrogen; then bubbles emerge from the end of the funnel, closed by porous vessel B
To understand it, consider the following analogy. When shooting at a moving target, in order to hit it, you have to aim at a point in front of the target. If you take aim at a target, then the bullets will hit behind the target. This deviation of the impact site from the target will be greater the faster the target moves and the lower the speed of the bullets.
The experiment of Otto Stern (1888–1969) was devoted to experimental confirmation and visualization of the velocity distribution of gas molecules. This is another beautiful experiment that made it possible to literally “draw” a graph of this distribution on an experimental setup. Stern's installation consisted of two rotating hollow cylinders with coinciding axes (see figure on the right; the large cylinder is not completely drawn). In the inner cylinder, a silver thread 1 was stretched directly along its axis, through which a current was passed, which led to its heating, partial melting and subsequent evaporation of silver atoms from its surface. As a result, the inner cylinder, which initially contained a vacuum, was gradually filled with gaseous silver of low concentration. In the inner cylinder, as shown in the figure, a thin slit 2 was made, so most of the silver atoms, reaching the cylinder, settled on it. A small part of the atoms passed through the gap and fell into the outer cylinder, in which a vacuum was maintained. Here these atoms no longer collided with other atoms and therefore moved in the radial direction at a constant speed, reaching the outer cylinder after a time inversely proportional to this speed:
where are the radii of the inner and outer cylinders, and is the radial component of the particle velocity. As a result, over time, a layer of silver coating appeared on the outer cylinder 3. In the case of cylinders at rest, this layer had the form of a strip located exactly opposite the slot in the inner cylinder. But if the cylinders rotated with the same angular velocity, then by the time the molecule reached the outer cylinder, the latter had already shifted by a distance
compared to the point directly opposite the slit (i.e., the point on which the particles settled in the case of stationary cylinders).
57. Derivation of the equation of state of an ideal gas (Mendeleev-Clayperon equation)
Gases are often reactants and products in chemical reactions. It is not always possible to get them to react with each other under normal conditions. Therefore, you need to learn how to determine the number of moles of gases under conditions other than normal.
To do this, use the ideal gas equation of state (also called the Clapeyron-Mendeleev equation): PV = nRT
where n is the number of moles of gas;
P – gas pressure (for example, in atm;
V – gas volume (in liters);
T – gas temperature (in kelvins);
R – gas constant (0.0821 l atm/mol K).
I found a derivation of the equation, but it is very complicated. We still have to look.
58. Isothermal process.
An isothermal process is a change in the state of a gas in which its temperature remains constant. An example of such a process is inflating car tires with air. However, such a process can be considered isothermal if we compare the state of the air before it enters the pump with its state in the tire after the temperature of the tire and the surrounding air have become equal. Any slow processes occurring with a small volume of gas surrounded by a large mass of gas, liquid or solid having a constant temperature can be considered isothermal.
In an isothermal process, the product of the pressure of a given mass of gas and its volume is a constant value. This law, called the Boyle-Mariotte law, was discovered by the English scientist R. Boyle and the French physicist E. Mariotte and is written as follows:
Find examples!
59. Isobaric process.
An isobaric process is a change in the state of a gas that occurs at constant pressure.
In an isobaric process, the ratio of the volume of a given mass of gas to its temperature is constant. This conclusion, which is called Gay-Lussac's law in honor of the French scientist J. Gay-Lussac, can be written as:
One example of an isobaric process is the expansion of small air and carbon dioxide bubbles contained in dough when it is placed in the oven. The air pressure inside and outside the oven is the same, and the temperature inside is approximately 50% higher than outside. According to Gay-Lussac's law, the volume of gas bubbles in the dough also increases by 50%, which makes the cake airy.
60. Isochoric process.
A process in which the state of a gas changes, but its volume remains unchanged, is called isochoric. From the Mendeleev-Clapeyron equation it follows that for a gas occupying a constant volume, the ratio of its pressure to temperature must also be constant:
Find examples!
61. Evaporation and condensation.
Vapor is a gas formed from molecules that have sufficient kinetic energy to escape a liquid.
We are accustomed to the fact that water and its steam can transform into each other. Puddles on the asphalt dry up after rain, and water vapor in the air often turns into tiny droplets of fog in the morning. All liquids have the ability to turn into vapor - to go into a gaseous state. The process of changing liquid into vapor is called evaporation. The formation of a liquid from its vapor is called condensation.
The molecular kinetic theory explains the evaporation process as follows. It is known (see §21) that an attractive force acts between liquid molecules, preventing them from moving away from each other, and the average kinetic energy of liquid molecules is not enough to overcome the adhesion forces between them. However, at any given moment of time, different molecules of a liquid have different kinetic energy, and the energy of some molecules can be several times higher than its average value. These high-energy molecules have a significantly higher speed of movement and therefore can overcome the attractive forces of neighboring molecules and fly out of the liquid, thus forming vapor above its surface (see Fig. 26a).
The molecules that make up the vapor that leave the liquid move randomly, colliding with each other in the same way as gas molecules do during thermal motion. At the same time, the chaotic movement of some vapor molecules can take them so far from the surface of the liquid that they never return there. Of course, the wind also contributes to this. On the contrary, the random movement of other molecules can lead them back into the liquid, which explains the process of vapor condensation.
Only molecules with kinetic energy much higher than the average can fly out of the liquid, which means that during evaporation the average energy of the remaining liquid molecules decreases. And since the average kinetic energy of the molecules of a liquid, like a gas (see 23.6), is proportional to temperature, during evaporation the temperature of the liquid decreases. That's why we get cold as soon as we leave the water, covered with a thin film of liquid, which immediately begins to evaporate and cool.
62. Saturated steam. Saturated vapor pressure.
What happens if a vessel with a certain volume of liquid is closed with a lid (Fig. 26b)? Every second, the fastest molecules will continue to leave the surface of the liquid, its mass will decrease, and the concentration of vapor molecules will increase. At the same time, some of its molecules will return to the liquid from the steam, and the greater the concentration of steam, the more intense this condensation process will be. Finally, the concentration of vapor above the liquid will become so high that the number of molecules returning to the liquid per unit time will become equal to the number of molecules leaving it. This state is called dynamic equilibrium, and the corresponding steam is called saturated steam. The concentration of vapor molecules above the liquid cannot be greater than their concentration in saturated vapor. If the concentration of vapor molecules is less than that of saturated vapor, then such vapor is called unsaturated.
Moving vapor molecules create pressure, the magnitude of which, as for a gas, is proportional to the product of the concentration of these molecules and the temperature. Therefore, at a given temperature, the higher the concentration of steam, the greater the pressure it exerts. Saturated vapor pressure depends on the type of liquid and temperature. The harder it is to tear the molecules of a liquid away from each other, the lower its saturated vapor pressure will be. Thus, the saturated vapor pressure of water at a temperature of 20 o C is about 2 kPa, and the saturated vapor pressure of mercury at 20 o C is only 0.2 Pa.
The life of humans, animals and plants depends on the concentration of water vapor (humidity) of the atmosphere, which varies widely depending on the place and time of year. Typically, the water vapor around us is unsaturated. Relative humidity is the ratio of water vapor pressure to saturated vapor pressure at the same temperature, expressed as a percentage. One of the instruments for measuring air humidity is a psychrometer, consisting of two identical thermometers, one of which is wrapped in a damp cloth.
63. Dependence of saturated vapor pressure on temperature.
Steam is a gas formed by evaporated molecules of a liquid, and therefore equation (23.7) is valid for it, relating the vapor pressure, p, the concentration of molecules in it, n and the absolute temperature, T:
From (27.1) it follows that the saturated vapor pressure should increase linearly with increasing temperature, as is the case for ideal gases in isochoric processes (see §25). However, as measurements have shown, the pressure of saturated vapor increases with temperature much faster than the pressure of an ideal gas (see Fig. 27a). This happens due to the fact that with increasing temperature, and therefore the average kinetic energy, more and more liquid molecules leave it, increasing the concentration n of vapor above it. And because according to (27.1) pressure is proportional to n, then this increase in vapor concentration explains the faster increase in saturated vapor pressure with temperature compared to an ideal gas. The increase in saturated vapor pressure with temperature explains the well-known fact that when heated, liquids evaporate faster. Note that as soon as the temperature rise leads to complete evaporation of the liquid, the vapor will become unsaturated.
When the liquid in each of the bubbles is heated, the evaporation process accelerates and the saturated vapor pressure increases. The bubbles expand and, under the influence of the buoyant force of Archimedes, break away from the bottom, float up and burst on the surface. In this case, the steam that filled the bubbles is carried away into the atmosphere.
The lower the atmospheric pressure, the lower the temperature this liquid boils at (see Fig. 27c). So, at the top of Mount Elbrus, where the air pressure is half the normal one, ordinary water boils not at 100 o C, but at 82 o C. On the contrary, if it is necessary to increase the boiling point of the liquid, then it is heated at increased pressure. This, for example, is the basis for the operation of pressure cookers, where food containing water can be cooked at a temperature of more than 100 o C without boiling.
64. Boiling.
Boiling is an intense evaporation process that occurs throughout the entire volume of a liquid and on its surface. A liquid begins to boil when its saturated vapor pressure approaches the pressure inside the liquid.
Boiling is the formation of a large number of vapor bubbles that float and burst on the surface of a liquid when it is heated. In fact, these bubbles are always present in the liquid, but their size increases and they become noticeable only when boiling. One of the reasons that there are always microbubbles in a liquid is as follows. A liquid, when it is poured into a vessel, displaces air from there, but cannot do this completely, and its small bubbles remain in microcracks and irregularities in the inner surface of the vessel. In addition, liquids usually contain microbubbles of steam and air stuck to tiny dust particles.
When the liquid in each of the bubbles is heated, the evaporation process accelerates and the saturated vapor pressure increases. The bubbles expand and, under the influence of the buoyant force of Archimedes, break away from the bottom, float up and burst on the surface. In this case, the steam that filled the bubbles is carried away into the atmosphere. Therefore, boiling is called evaporation, which occurs throughout the entire volume of the liquid. Boiling begins at the temperature when gas bubbles are able to expand, and this occurs if the saturated vapor pressure exceeds atmospheric pressure. Thus, the boiling point is the temperature at which the saturated vapor pressure of a given liquid is equal to atmospheric pressure. While the liquid boils, its temperature remains constant.
The boiling process is impossible without the participation of the Archimedean buoyancy force. Therefore, at space stations in conditions of weightlessness there is no boiling, and heating of water only leads to an increase in the size of steam bubbles and their combination into one large steam bubble inside a vessel with water.
65. Critical temperature.
There is also such a concept as critical temperature; if a gas is at a temperature above the critical temperature (individual for each gas, for example for carbon dioxide approximately 304 K), then it can no longer be turned into liquid, no matter what pressure is applied to it. This phenomenon occurs due to the fact that at a critical temperature the surface tension forces of the liquid are zero.
Table 23. Critical temperature and critical pressure of some substances
What does the existence of a critical temperature indicate? What happens at even higher temperatures?
Experience shows that at temperatures higher than critical, a substance can only be in a gaseous state.
The existence of a critical temperature was first pointed out in 1860 by Dmitry Ivanovich Mendeleev.
After the discovery of the critical temperature, it became clear why gases such as oxygen or hydrogen could not be converted into liquid for a long time. Their critical temperature is very low (Table 23). To turn these gases into liquid, they must be cooled below a critical temperature. Without this, all attempts to liquefy them are doomed to failure.
66. Partial pressure. Relative humidity. 67. Instruments for measuring relative air humidity.
The life of humans, animals and plants depends on the concentration of water vapor (humidity) of the atmosphere, which varies widely depending on the place and time of year. Typically, the water vapor around us is unsaturated. Relative humidity is the ratio of water vapor pressure to saturated vapor pressure at the same temperature, expressed as a percentage. One of the instruments for measuring air humidity is a psychrometer, consisting of two identical thermometers, one of which is wrapped in a damp cloth. When the air humidity is less than 100%, the water from the cloth will evaporate, and thermometer B will cool, showing a lower temperature than A. And the lower the air humidity, the greater the difference, Dt, between the readings of thermometers A and B. Using a special psychrometric table, the air humidity can be determined from this temperature difference.
Partial pressure is the pressure of a certain gas included in a gas mixture, which this gas would exert on the walls of the container containing it if it alone occupied the entire volume of the mixture at the temperature of the mixture.
Partial pressure is not measured directly, but is estimated based on the total pressure and composition of the mixture.
Gases dissolved in water or body tissue also exert pressure because the dissolved gas molecules are in random motion and have kinetic energy. If a gas dissolved in a liquid hits a surface, such as a cell membrane, it exerts a partial pressure in the same way as a gas in a gas mixture.
Pressure pressure cannot be measured directly; it is calculated based on the total pressure and composition of the mixture.
Factors that determine the magnitude of the partial pressure of a gas dissolved in a liquid. The partial pressure of a gas in a solution is determined not only by its concentration, but also by its solubility coefficient, i.e. Some types of molecules, such as carbon dioxide, are physically or chemically attached to water molecules, while others are repelled. This relationship is called Henry's law and is expressed by the following formula: Partial pressure = Dissolved gas concentration / Solubility coefficient.
68. Surface tension.
The most interesting feature of liquids is the presence of a free surface. Liquid, unlike gases, does not fill the entire volume of the container into which it is poured. An interface is formed between liquid and gas (or vapor), which is in special conditions compared to the rest of the liquid. Molecules in the boundary layer of a liquid, unlike molecules in its depth, are not surrounded by other molecules of the same liquid on all sides. The forces of intermolecular interaction acting on one of the molecules inside a liquid from neighboring molecules are, on average, mutually compensated. Any molecule in the boundary layer is attracted by molecules located inside the liquid (the forces acting on a given liquid molecule from gas (or vapor) molecules can be neglected). As a result, a certain resultant force appears, directed deep into the liquid. Surface molecules are drawn into the liquid by forces of intermolecular attraction. But all molecules, including molecules of the boundary layer, must be in a state of equilibrium. This equilibrium is achieved by slightly reducing the distance between the molecules of the surface layer and their nearest neighbors inside the liquid. As can be seen from Fig. 3.1.2, when the distance between molecules decreases, repulsive forces arise. If the average distance between molecules inside the liquid is equal to r0, then the molecules of the surface layer are packed somewhat more densely, and therefore they have an additional supply of potential energy compared to the internal molecules (see Fig. 3.1.2). It should be borne in mind that due to the extremely low compressibility, the presence of a more densely packed surface layer does not lead to any noticeable change in the volume of the liquid. If a molecule moves from the surface into the liquid, the forces of intermolecular interaction will do positive work. On the contrary, in order to pull a certain number of molecules from the depths of the liquid to the surface (i.e., increase the surface area of the liquid), external forces must perform positive work ΔAext, proportional to the change ΔS of the surface area: ΔAext = σΔS.
The coefficient σ is called the surface tension coefficient (σ > 0). Thus, the coefficient of surface tension is equal to the work required to increase the surface area of a liquid at constant temperature by one unit.
In SI, the coefficient of surface tension is measured in joules per square meter (J/m2) or in newtons per meter (1 N/m = 1 J/m2).
It is known from mechanics that the equilibrium states of a system correspond to the minimum value of its potential energy. It follows that the free surface of the liquid tends to reduce its area. For this reason, a free drop of liquid takes on a spherical shape. The liquid behaves as if forces acting tangentially to its surface are contracting (pulling) this surface. These forces are called surface tension forces.
The presence of surface tension forces makes the surface of a liquid look like an elastic stretched film, with the only difference that the elastic forces in the film depend on its surface area (i.e., on how the film is deformed), and the surface tension forces do not depend on the surface area liquids.
Some liquids, such as soapy water, have the ability to form thin films. Well-known soap bubbles have a regular spherical shape - this also shows the effect of surface tension forces. If you lower a wire frame, one of the sides of which is movable, into a soap solution, then the entire frame will be covered with a film of liquid.
69. Wetting.
Everyone knows that if you place a drop of liquid on a flat surface, it will either spread across it or take on a round shape. Moreover, the size and convexity (the value of the so-called contact angle) of a lying drop is determined by how well it wets a given surface. The phenomenon of wetting can be explained as follows. If the molecules of a liquid are attracted to each other more than to the molecules of a solid, the liquid tends to form a droplet.
An acute contact angle occurs on a wettable (lyophilic) surface, while an obtuse contact angle occurs on a non-wettable (lyophobic) surface.
This is how mercury behaves on glass, water on paraffin or on a “greasy” surface. If, on the contrary, the molecules of a liquid are attracted to each other less strongly than to the molecules of a solid, the liquid is “pressed” to the surface and spreads over it. This happens with a drop of mercury on a zinc plate or with a drop of water on clean glass. In the first case, they say that the liquid does not wet the surface (contact angle is greater than 90°), and in the second case, it wets it (contact angle is less than 90°).
It is the water-repellent lubricant that helps many animals escape from excessive wetness. For example, studies of marine animals and birds - fur seals, seals, penguins, loons - have shown that their downy hair and feathers have hydrophobic properties, while the guard hairs of animals and the upper part of the contour feathers of birds are well wetted by water. As a result, an air layer is created between the animal’s body and the water, which plays a significant role in thermoregulation and thermal insulation.
But lubrication isn't everything. The surface structure also plays a significant role in the wetting phenomenon. Rough, bumpy or porous terrain can improve wetting. Let us recall, for example, sponges and terry towels, which perfectly absorb water. But if the surface is initially “afraid” of water, then the developed relief will only aggravate the situation: droplets of water will collect on the ledges and roll down.
70. Capillary phenomena.
Capillary phenomena are the rise or fall of liquid in small-diameter tubes - capillaries. Wetting liquids rise through the capillaries, non-wetting liquids descend.
In Fig. Figure 3.5.6 shows a capillary tube of a certain radius r, lowered at its lower end into a wetting liquid of density ρ. The upper end of the capillary is open. The rise of the liquid in the capillary continues until the force of gravity acting on the column of liquid in the capillary becomes equal in magnitude to the resulting Fн surface tension forces acting along the boundary of contact of the liquid with the surface of the capillary: Fт = Fн, where Fт = mg = ρhπr2g, Fн = σ2πr cos θ.
This implies:
Figure 3.5.6.
Rise of the wetting fluid in the capillary.
With complete wetting θ = 0, cos θ = 1. In this case
With complete non-wetting θ = 180°, cos θ = –1 and, therefore, h< 0. Уровень несмачивающей жидкости в капилляре опускается ниже уровня жидкости в сосуде, в которую опущен капилляр.
Water almost completely wets the clean glass surface. On the contrary, mercury does not completely wet the glass surface. Therefore, the level of mercury in the glass capillary drops below the level in the vessel.
71. Crystalline bodies and their properties.
Unlike liquids, a solid retains not only its volume, but also its shape and has significant strength.
The variety of solids encountered can be divided into two groups that differ significantly in their properties: crystalline and amorphous.
Basic properties of crystalline bodies
1. Crystalline bodies have a certain melting temperature tmelt, which does not change during the melting process at constant pressure (Fig. 1, curve 1).
2. Crystalline bodies are characterized by the presence of a spatial crystal lattice, which is an ordered arrangement of molecules, atoms or ions, repeated throughout the entire volume of the body (long-range order). Any crystal lattice is characterized by the existence of such an element of its structure, the repeated repetition of which in space can produce the entire crystal. This is a single crystal. A polycrystal consists of many very small single crystals fused together, which are randomly oriented in space.
§ 12th. Motion with constant acceleration
For uniformly accelerated motion, the following equations are valid, which we present without derivation:
As you understand, the vector formula on the left and the two scalar formulas on the right are equal. From an algebraic point of view, scalar formulas mean that with uniformly accelerated motion, the displacement projections depend on time according to a quadratic law. Compare this with the nature of instantaneous velocity projections (see § 12-h).
Knowing that s x = x – x o And s y = y – y o(see § 12), from the two scalar formulas from the upper right column we obtain equations for coordinates:
Since the acceleration during uniformly accelerated motion of a body is constant, the coordinate axes can always be positioned so that the acceleration vector is directed parallel to one axis, for example the Y axis. Consequently, the equation of motion along the X axis will be noticeably simplified:
x = x o + υ ox t + (0) And y = y o + υ oy t + ½ a y t²
Please note that the left equation coincides with the equation of uniform rectilinear motion (see § 12-g). It means that uniformly accelerated motion can “compose” from uniform motion along one axis and uniformly accelerated motion along the other. This is confirmed by the experience with the core on a yacht (see § 12-b).
Task. Stretching out her arms, the girl tossed the ball. He rose 80 cm and soon fell at the girl’s feet, flying 180 cm. At what speed was the ball thrown and what speed did the ball have when it hit the ground?
Let's square both sides of the equation to project the instantaneous velocity onto the Y axis: υ y = υ oy + a y t(see § 12). We get the equality:
υ y ² = ( υ oy + a y t )² = υ oy ² + 2 υ oy a y t + a y ² t²
Let's take the factor out of brackets 2 a y only for the two right-hand terms:
υ y ² = υ oy ² + 2 a y ( υ oy t + ½ a y t² )
Note that in brackets we get the formula for calculating the displacement projection: s y = υ oy t + ½ a y t². Replacing it with s y, we get:
Solution. Let's make a drawing: direct the Y axis upward, and place the origin of coordinates on the ground at the girl's feet. Let us apply the formula we derived for the square of the velocity projection, first at the top point of the ball’s rise:

0 = υ oy ² + 2·(–g)·(+h) ⇒ υ oy = ±√¯2gh = +4 m/s
Then, when starting to move from the top point down:
υ y² = 0 + 2·(–g)·(–H) ⇒ υ y = ±√¯2gh = –6 m/s
Answer: the ball was thrown upward with a speed of 4 m/s, and at the moment of landing it had a speed of 6 m/s, directed against the Y axis.
Note. We hope you understand that the formula for the square of the projection of instantaneous velocity will be correct by analogy for the X axis.
In this lesson, the topic of which is: “Equation of motion with constant acceleration. Forward movement,” we will remember what movement is, what it happens. Let’s also remember what acceleration is, consider the equation of motion with constant acceleration and how to use it to determine the coordinates of a moving body. Let's consider an example of a task for consolidating material.
The main task of kinematics is to determine the position of the body at any time. The body can be at rest, then its position will not change (see Fig. 1).
Rice. 1. Body at rest
A body can move in a straight line at a constant speed. Then its movement will change uniformly, that is, equally over equal periods of time (see Fig. 2).

Rice. 2. Movement of a body when moving at a constant speed
Movement, speed multiplied by time, we have been able to do this for a long time. A body can move with constant acceleration; consider such a case (see Fig. 3).

Rice. 3. Body motion with constant acceleration
Acceleration
|
Acceleration is the change in speed per unit time(see Fig. 4) :
Rice. 4. Acceleration Speed is a vector quantity, therefore the change in speed, i.e. the difference between the vectors of the final and initial speed, is a vector. Acceleration is also a vector, directed in the same direction as the vector of the speed difference (see Fig. 5).
We are considering linear motion, so we can select a coordinate axis along the straight line along which the motion occurs, and consider the projections of the velocity and acceleration vectors onto this axis:
|
Then its speed changes uniformly: (if its initial speed was zero). How to find the displacement now? It is impossible to multiply speed by time: the speed was constantly changing; which one to take? How to determine where the body will be at any moment during such a movement - today we will solve this problem.
Let’s immediately define the model: we are considering the rectilinear translational motion of a body. In this case, we can use the material point model. Acceleration is directed along the same straight line along which the material point moves (see Fig. 6).

Forward movement
|
Translational motion is a movement in which all points of the body move the same way: at the same speed, making the same movement (see Fig. 7).
Rice. 7. Forward movement How else could it be? Wave your hand and observe: it is clear that the palm and shoulder moved differently. Look at the Ferris wheel: the points near the axis hardly move, but the cabins move at different speeds and along different trajectories (see Fig. 8).
Rice. 8. Movement of selected points on the Ferris wheel Look at a moving car: if you do not take into account the rotation of the wheels and the movement of engine parts, all points of the car move equally, we consider the movement of the car to be translational (see Fig. 9).
Rice. 9. Car movement Then there is no point in describing the movement of each point; you can describe the movement of one. We consider a car to be a material point. Please note that during translational movement, the line connecting any two points of the body during movement remains parallel to itself (see Fig. 10).
Rice. 10. Position of the line connecting two points |
The car drove straight for an hour. At the beginning of the hour his speed was 10 km/h, and at the end - 100 km/h (see Fig. 11).

Rice. 11. Drawing for the problem
The speed changed uniformly. How many kilometers did the car travel?
Let us analyze the condition of the problem.
The speed of the car changed uniformly, that is, its acceleration was constant throughout the journey. Acceleration by definition is equal to:
![]()
The car was driving straight, so we can consider its movement in projection onto one coordinate axis:
![]()
Let's find the displacement.
Increasing speed example
|
Nuts are placed on the table, one nut per minute. It’s clear: no matter how many minutes pass, so many nuts will appear on the table. Now let’s imagine that the rate of placing nuts increases uniformly from zero: the first minute no nuts are placed, the second minute they put one nut, then two, three, and so on. How many nuts will be on the table after some time? It is clear that it is less than if the maximum speed was always maintained. Moreover, it is clearly visible that it is 2 times less (see Fig. 12).
Rice. 12. Number of nuts at different laying speeds It’s the same with uniformly accelerated motion: let’s say that at first the speed was zero, but at the end it became equal (see Fig. 13).
Rice. 13. Change speed If the body were constantly moving at such a speed, its displacement would be equal to , but since the speed increased uniformly, it would be 2 times less. |
We know how to find displacement during UNIFORM movement: . How to work around this problem? If the speed does not change much, then the movement can be approximately considered uniform. The change in speed will be small over a short period of time (see Fig. 14).

Rice. 14. Change speed
Therefore, we divide the travel time T into N small segments of duration (see Fig. 15).

Rice. 15. Splitting a period of time
Let's calculate the displacement at each time interval. The speed increases at each interval by:
On each segment we will consider the movement to be uniform and the speed approximately equal to the initial speed for a given period of time. Let's see if our approximation will lead to an error if we assume the motion to be uniform over a short interval. The maximum error will be:
and the total error for the entire journey -> . For large N we assume the error is close to zero. We will see this on the graph (see Fig. 16): there will be an error at each interval, but the total error with a sufficiently large number of intervals will be negligible.

Rice. 16. Interval error
So, each subsequent speed value is the same amount greater than the previous one. From algebra we know that this is an arithmetic progression with a progression difference:
The path in the sections (with uniform rectilinear motion (see Fig. 17) is equal to:

Rice. 17. Consideration of areas of body movement
On the second section:
On the nth section the path is:
Arithmetic progression
|
Arithmetic progression is a number sequence in which each subsequent number differs from the previous one by the same amount. An arithmetic progression is specified by two parameters: the initial term of the progression and the difference of the progression. Then the sequence is written like this: The sum of the first terms of an arithmetic progression is calculated using the formula:
|
Let's sum up all the paths. This will be the sum of the first N terms of the arithmetic progression:
Since we have divided the movement into many intervals, we can assume that then:
We had many formulas, and in order not to get confused, we did not write the x indices each time, but considered everything in projection onto the coordinate axis.
So, we have obtained the main formula for uniformly accelerated motion: displacement during uniformly accelerated motion in time T, which, along with the definition of acceleration (change in speed per unit time), we will use to solve problems:
![]()
![]()
We were working on solving a problem about a car. Let's substitute numbers into the solution and get the answer: the car traveled 55.4 km.
Mathematical part of solving the problem
We figured out the movement. How to determine the coordinate of a body at any moment in time?
By definition, the movement of a body over time is a vector, the beginning of which is at the initial point of movement, and the end is at the final point at which the body will be after time. We need to find the coordinate of the body, so we write an expression for the projection of displacement onto the coordinate axis (see Fig. 18):

Rice. 18. Motion projection
Let's express the coordinate:
That is, the coordinate of the body at the moment of time is equal to the initial coordinate plus the projection of the movement that the body made during the time. We have already found the projection of displacement during uniformly accelerated motion, all that remains is to substitute and write:
![]()
This is the equation of motion with constant acceleration. It allows you to find out the coordinates of a moving material point at any time. It is clear that we choose the moment of time within the interval when the model works: the acceleration is constant, the movement is rectilinear.
Why the equation of motion cannot be used to find a path
|
In what cases can we consider movement modulo equal to path? When a body moves along a straight line and does not change direction. For example, with uniform rectilinear motion, we do not always clearly define whether we are finding a path or a displacement; they still coincide. With uniformly accelerated motion, the speed changes. If the speed and acceleration are directed in opposite directions (see Fig. 19), then the velocity modulus decreases, and at some point it will become equal to zero and the speed will change direction, that is, the body will begin to move in the opposite direction.
Rice. 19. Velocity modulus decreases And then, if at a given moment in time the body is at a distance of 3 m from the beginning of observation, then its displacement is equal to 3 m, but if the body first traveled 5 m, then turned around and traveled another 2 m, then the path will be equal to 7 m. And how How can you find it if you don’t know these numbers? You just need to find the moment when the speed is zero, that is, when the body turns around, and find the path to and from this point (see Fig. 20).
Rice. 20. The moment when the speed is 0 |
Bibliography
- Sokolovich Yu.A., Bogdanova G.S. Physics: A reference book with examples of problem solving. - 2nd edition repartition. - X.: Vesta: Ranok Publishing House, 2005. - 464 p.
- Landsberg G.S. Elementary physics textbook; v.1. Mechanics. Heat. Molecular physics - M.: Publishing house "Science", 1985.
- Internet portal “kaf-fiz-1586.narod.ru” ()
- Internet portal “Study - Easy” ()
- Internet portal "Knowledge Hypermarket" ()
Homework
- What is an arithmetic progression?
- What kind of movement is called translational?
- What is a vector quantity characterized by?
- Write down the formula for acceleration through a change in speed.
- What is the form of the equation of motion with constant acceleration?
- The acceleration vector is directed towards the movement of the body. How will the body change its speed?